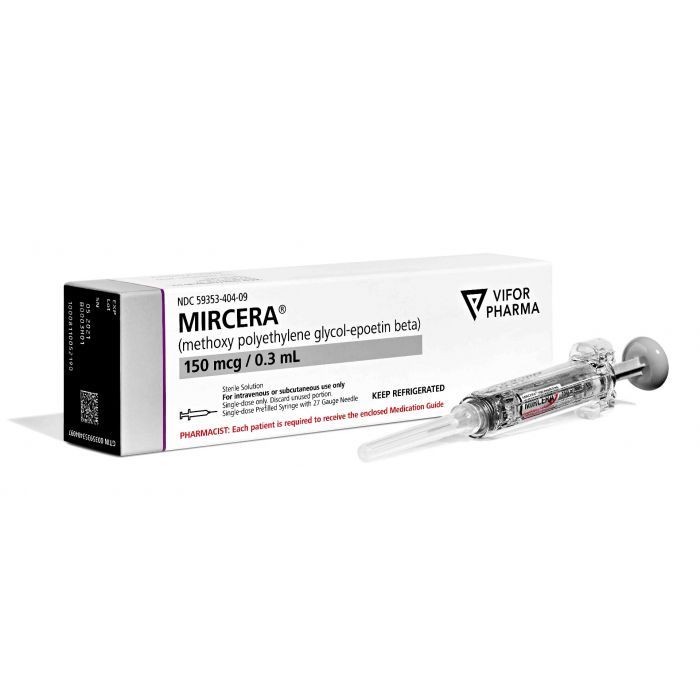
What is Mircera (methoxy polyethylene glycol-epoetin beta) for?
Mircera (methoxy polyethylene glycol-epoetin beta) is a medicine used to treat anemia caused by chronic kidney disease (CKD) in adults, or in children at least 5 years old who are on dialysis (a blood clearance technique used in advanced kidney disease).[1]
It is available in syringe form containing 30 mcg, 50 mcg, 75 mcg, 100 mcg, 120 mcg, 150 mcg, 200 mcg, or 250 mcg in 0.3 mL solution or 360 mcg in 0.6 mL solution methoxy polyethylene glycol-epoetin beta.[1]
How does Mircera (methoxy polyethylene glycol-epoetin beta) work?
The hormone erythropoietin stimulates the production of red blood cells from the bone marrow. The kidneys produce erythropoietin. However, in patients with CKD, a lack of natural erythropoietin causes anemia.[2,3]
The active ingredient in Mircera, methoxy polyethylene glycol-epoetin beta, is a is a long-acting erythropoiesis-stimulating agent (ESA). It stimulates red blood cell production. It does so because it can attach itself to the same receptors as erythropoietin.[2,3]
However, because it interacts with the receptor slightly differently from erythropoietin made by the body itself, its effect lasts longer. It is also cleared from the body less quickly. As a result, Mircera can be given less often than natural erythropoietin.[2,3]
Where has Mircera (methoxy polyethylene glycol-epoetin beta) been approved?
Mircera (methoxy polyethylene glycol-epoetin beta) was approved by:
- The European Medicines Agency (EMA), Europe:
- July 20, 2007 for the treatment of anaemia associated with CKD.[3]
- Swissmedic, Switzerland:
- September 2007 for the treatment of anemia associated with CKD.
- The Food and Drug Administration (FDA), USA:[4]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Mircera (methoxy polyethylene glycol-epoetin beta) taken?
Mircera (methoxy polyethylene glycol-epoetin beta) can be injected under the skin (subcutaneous) or into a vein (intravenous). The dose and the frequency of dosing depend on whether or not Mircera is replacing another ESA.[1]
Doses should be adjusted according to the patient’s response, to obtain Hb levels within the recommended range (between 10-12 g/dl). Hb levels should be monitored every two weeks until they are stable, and then periodically. The lowest dose that provides adequate control of symptoms should be used.[1]
Mircera is intended for long-term use. Once they have been trained appropriately, patients can inject themselves.[1]
Complete information about Mircera (methoxy polyethylene glycol-epoetin beta) dosage and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Mircera (methoxy polyethylene glycol-epoetin beta)?
Common adverse reactions
The most common side effects ( ≥10% of patients) listed in the prescribing information include:[1]
- Hypertension (high blood pressure)
- Diarrhea
- Nasopharyngitis (cold)
- Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Increased risk of heart problems
- Seizures
- Pure red cell aplasia (PRCA)
- Serious allergic reactions
- Severe skin reactions






Reviews
There are no reviews yet.