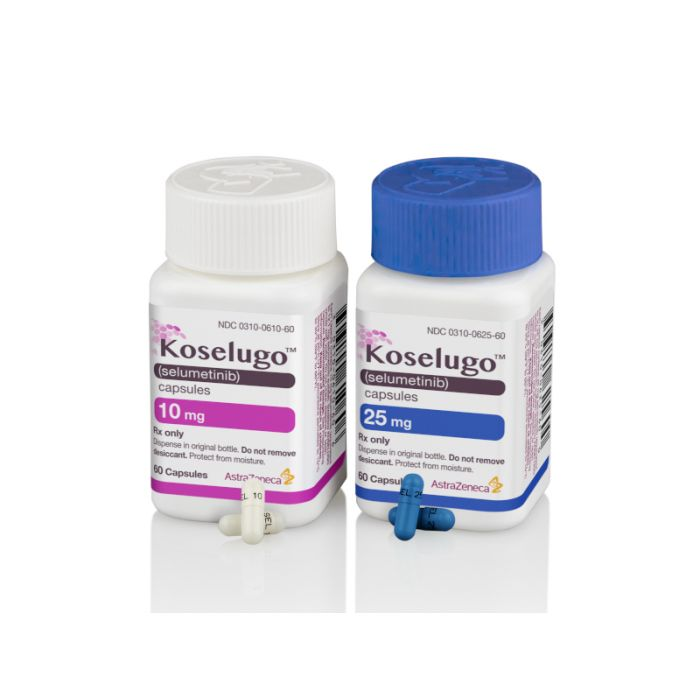
What is Koselugo (selumetinib) for?
Koselugo (selumetinib) is an inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2; kinase inhibitor) for pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas.[1]
It is available in capsule form each containing 10 mg or 25 mg selumetinib.[1]
How does Koselugo (selumetinib) work?
NF1 is a rare genetic disorder that is characterized by tumor growth along nerves in the skin, brain and other parts of the body.[2]
NF1 is caused by a mutation in the NF1 gene. This gene is responsible for the production of the protein neurofibromin. This protein, naturally produced in nerve cells and cells surrounding nerves, works as a tumor suppressor. It inhibits the function of RAS, a protein that promotes tumor growth. Neurofibromin stops cells from growing and dividing too fast or uncontrollably.[2]
Mutations in the NF1 gene lead to the making of a dysfunctional neurofibromin protein. It cannot control cell growth anymore, and as a result, nerve cells and cells along the nerves will form tumors. Some NF1 patients develop benign plexiform neurofibromas that can lead to disfigurement and have deadly, compressing effects.[3]
Selumetinib is an oral inhibitor of the protein MEK1/2, which is a target of RAS. By inhibiting these proteins, selumetinib can help stop the tumor cells from growing.[3]
Where has Koselugo (selumetinib) been approved?
Koselugo (selumetinib) was approved for the treatment of pediatric patients, 2 years of age and older, with NF1 by:
- The Food and Drugs Administration (FDA), USA on April 10, 2020.[4]
The FDA granted Koselugo (selumetinib) Priority Review and Breakthrough Therapy designation. Koselugo also received Orphan Drug and Rare Pediatric Disease designation, intended to encourage the development of drugs that fulfill an unmet need.[4]
Koselugo (selumetinib) is the first drug approved by the FDA to treat NF1.[4]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Koselugo (selumetinib) taken?
The standard dosage is:[1]
- 25 mg/m2 body surface area
Koselugo (selumetinib) is taken orally twice daily (approximately every 12 hours).
The average body surface area for adult men: 1.9 m2. Average body surface area for adult women: 1.6 m2. Average body surface area for children (9 years): 1.07 m2.
Dosage should be modified when patients experience adverse reactions.
Complete information about Koselugo (selumetinib) dosage, dose reduction and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing.
Are there any known adverse reactions or side effects of Koselugo?
Common adverse reactions
The most common adverse reactions (≥20% of patients) listed in the prescribing information include:[1]
- Nausea
- Vomiting
- Stomach pain
- Itching
- Dry skin
- Acne
- Redness around the fingernails
- Feeling weak or tired
- Mouth pain or soreness, swollen gums
- Muscle or bone pain
- Headache
- Fever
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Heart problems
- Eye problems
- Severe diarrhea
- Skin rash
- Muscle problems






Reviews
There are no reviews yet.