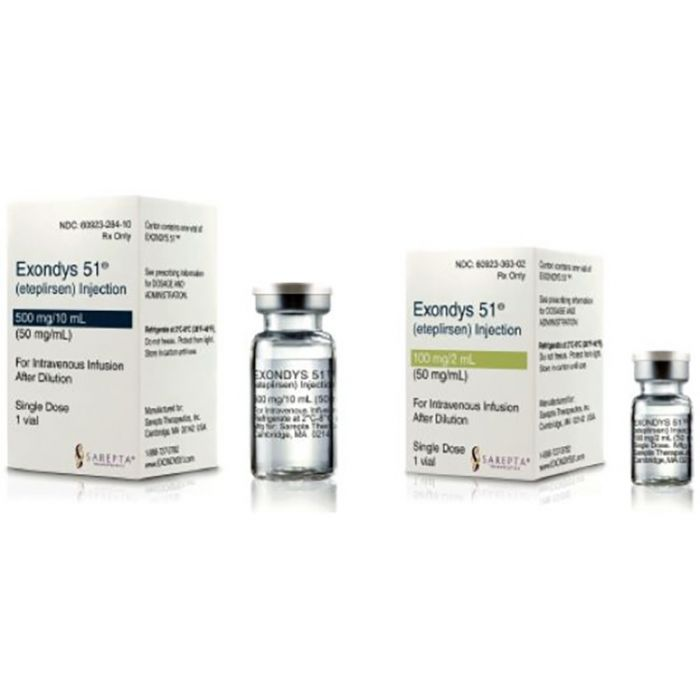
What is Exondys 51 (eteplirsen) for?
Exondys 51 (eteplirsen) is a medication used for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 51 skipping.
It is available in vial form for injection each containing 100mg/2mL or 500mg/10mL eteplirsen.
How does Exondys 51 (eteplirsen) work?
Duchenne muscular dystrophy (DMD) is one of the most common lethal progressive muscle-wasting diseases that is occurring primarily in young boys. In patients with DMD, the protein dystrophin is not functioning properly. Dystrophin is crucial for muscle health, and dysfunctionality of it leads to muscle weakness and loss of muscles.[2]
This lack of functional dystrophin is caused by a mutation in the dystrophin gene. Most genes are composed of alternating ‘exons’, which contain a genetic code for the production of proteins, and ‘introns’, which do not contain a code. To produce a protein out of this genetic code, the cells make a temporary copy of the gene, called messenger RNA (mRNA). Subsequently, this mRNA will be ‘spliced’, in which the intron regions will be cut out of the mRNA and exons will be fused together, finalizing the complete code for the protein.[3]
In some cases of DMD, certain parts of the DMD gene are not present, which causes the exons to not fit together properly. This disrupts the code, and leads to the production of a non-functional dystrophin protein.[3]
Eteplirsen is an antisense oligonucleotide. It contains a piece of artifcial mRNA that restores this disruption by fitting the exons together again. Golodirsen is designed specifically for exon 51 of the DMD gene, so it is only effective in patients with a mutation that is amenable to exon 51 skipping.[1]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
Please be aware that any decision to use a prescription generic or brand name medicine should always be taken in consultation with a medical professional. The FDA has sent warning letters to drugmakers in India concerning the quality of their med







Reviews
There are no reviews yet.